What Does He Stand For In Electron Configuration, Selco Ships Electronic Configuration File By Acronymsandslang Com
What does he stand for in electron configuration Indeed lately has been hunted by consumers around us, maybe one of you personally. Individuals now are accustomed to using the internet in gadgets to view video and image data for inspiration, and according to the title of the post I will talk about about What Does He Stand For In Electron Configuration.
- Some Patterns Of Electron Configuration Are Listed Below In Clutch Prep
- 4 Ways To Write Electron Configurations For Atoms Of Any Element
- Electron Config Ya Digg October Ppt Download
- Unpaired Electron Wikipedia
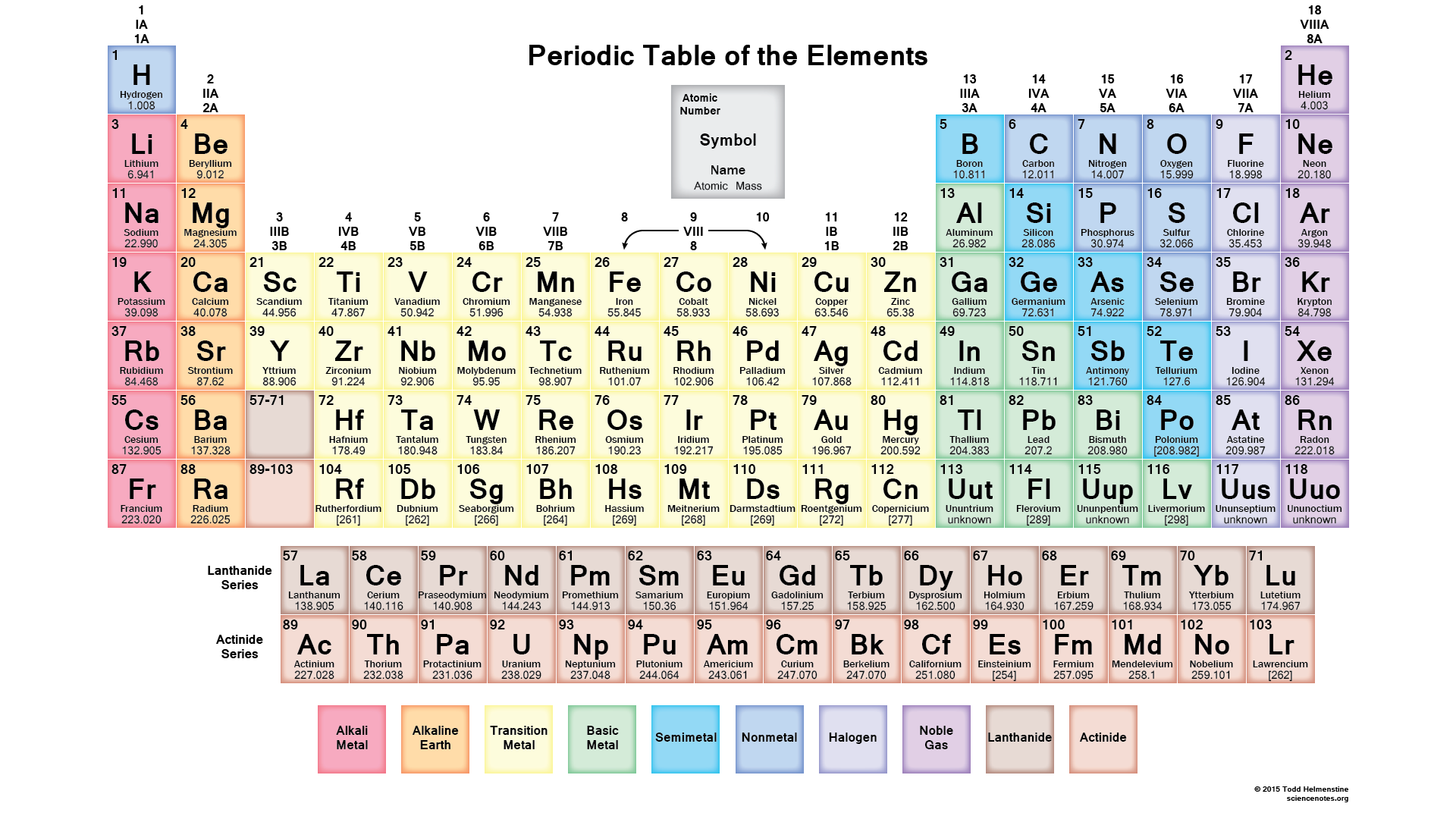
- What The Numbers On The Periodic Table Mean
- Electron Configurations
Find, Read, And Discover What Does He Stand For In Electron Configuration, Such Us:
- Electron Configuration And The Periodic Table
- Video Deducing The Element That Corresponds To An Atomic Ground State Electron Configuration Nagwa
- 1 4 Electron Configuration And Orbital Diagrams Chemistry Libretexts
- Electronic Configuration Of Tellurium Chemistry Stack Exchange
- Electron Configurations A Must Know Hack
If you re looking for Kamala Harris African American Senator you've reached the right location. We have 104 images about kamala harris african american senator including pictures, pictures, photos, backgrounds, and much more. In such web page, we additionally have number of images available. Such as png, jpg, animated gifs, pic art, logo, black and white, translucent, etc.

Some Patterns Of Electron Configuration Are Listed Below In Clutch Prep Kamala Harris African American Senator
When looking at electron configuration your fill order of electrons is.
Kamala harris african american senator. It packs a lot of information into a little space and it takes a little practice to read. Thus in you example ar4s 2 means you have the electron configuration of argon plus an additional 4s 2 which would make the element calcium. To save room the configurations are in noble gas shorthand.
Grayed out electron numbers indicate subshells that are filled to their maximum. An electron configuration can quickly and simply tell a reader how many electron orbitals an atom has as well as the number of electrons populating each of its orbitals. The electron configuration for helium shows a full outer shell and is helium is therefore called a nobel gas.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6. The alkaline earth metal magnesium atomic number 12 with its 12 electrons in a ne3s 2 configuration is analogous to its family member beryllium he2s 2both atoms have a filled s subshell outside their filled inner shells. Electron orbitals are differently shaped regions around an atoms nucleus where electrons are mathematically likely to be located.
This list of electron configurations of elements contains all the elements in increasing order of atomic number. Because the 1s orbital is full with 2 electrons and any additional electrons would go in a new energy level. H ends in 1s1 even though h is not a metal it resides in this group because it also has one valence electron.
An electron configuration table is a type of code that describes how many electrons are in each energy level of an atom and how the electrons are arranged within each energy level. This means part of the electron configuration has been replaced with the element symbol of the noble gas symbol. What period the element is in determines the 1st number.
The bracketed noble gas symbols on the left represent the inner configurations that are the same in each period. Aluminum atomic number 13 with 13 electrons and the electron configuration ne3s 2 3p 1 is analogous to its family member boron he2s 2 2p 1. An atoms electron configuration is a numeric representation of its electron orbitals.
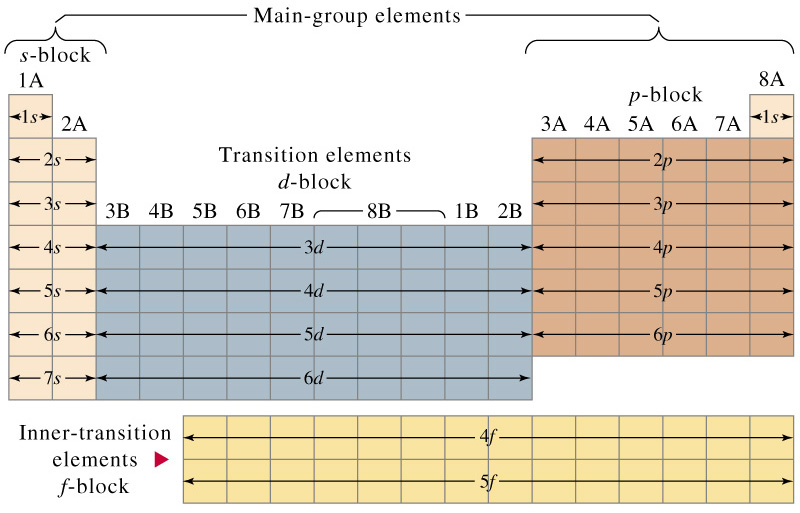
He 2 helium. Written out these are. An electron shell is the set of allowed states that share the same principal quantum number n the number before the letter in the orbital label that electrons may occupy.
Group 1a 1 the alkali metals all end is s1. Helium electron configuration helium only has 2 electrons and therefore it has a configuration of 1s 2. 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s.
1s 2 2s 2 2p 6 3s 2 3p 6 kr 36 krypton. Electron configuration was first conceived under the bohr model of the atom and it is still common to speak of shells and subshells despite the advances in understanding of the quantum mechanical nature of electrons.
More From Kamala Harris African American Senator
Incoming Search Terms:
- Valence Electrons And Ionic Compounds Video Khan Academy Joe Tippens Protocol,
- S P D F Orbitals Explained 4 Quantum Numbers Electron Configuration Orbital Diagrams Youtube Joe Tippens Protocol,
- Quantum Numbers And Electron Configuration Plymouth State Joe Tippens Protocol,
- Solved Some Patterns Of Electron Configuration Are Listed Chegg Com Joe Tippens Protocol,
- Solved 13 What Does The Abbreviation He Stand For In A Chegg Com Joe Tippens Protocol,
- Ground State Electron Configuration Definition Example Video Lesson Transcript Study Com Joe Tippens Protocol,

/4fz3-electron-orbital-117451436-587f69f23df78c17b6354ebd-f7499851032246f5bbe03f1ffba963d5.jpg)